How To Draw A Tetrahedral Molecule
4.9: Three Dimensional Drawings
- Folio ID
- 191163
Our purpose in this chapter is to be able to correspond the structure of a molecule. When we draw the Lewis structure for methane, it is only a 2-dimensional representation, but really the chemical compound has a three-dimensional shape. The central carbon in methane is tetrahedral, with the four hydrogens at the alternate corners of a cube.
We can draw a tetrahedron past making a vertex betwixt two lines, and so attaching a wedged line and a hatched line to the outside of the corner. The black, wedged line is read every bit "this atom is towards us". The hatched line is read equally "this atom is farther away from u.s.". Although the drawing is apartment, the hatched and wedged lines is a shorthand notation for three-dimensionality.
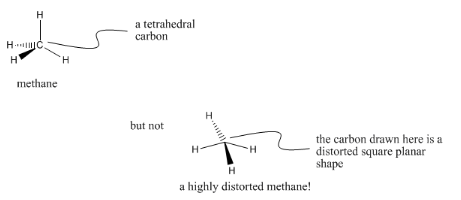
Representing the 3-dimensional shape of a molecule tin exist done on paper, but only if we are very careful about drawing conventions such as wedge-dash drawings.
Carbons that have bonds to four dissimilar neighbors are always tetrahedral. The neighbors bundled around a tetrahedral carbon are effectually 109° apart. Sometimes, for reasons we won't go into hither, tetrahedral carbons are called sp3 carbons.
Pentane is a chemical compound that contains four tetrahedral carbons in a row. Each tetrahedral carbon could be shown with wedge - nuance projections.
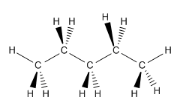
It is also very common to use computer drawing programs to testify the shapes of molecules. Yous tin view the three-dimensional structure of pentane in many different ways. Three of the most common ways are shown hither. Ball & Stick is an piece of cake way to see where all the atoms are and how they are connected. Wireframe allows you to see connections without having your view obscured by atoms. Space-filling models are meant to about closely bear witness you the shape of the molecule, although the atoms involved become obscured in this view.
Go to Animation IM9.ane. A 3-dimensional model of pentane.
Carbons double bonded to one neighbor, and consequently bonded to three different neighbors overall, are trigonal planar. The neighbors are all in the aforementioned plane and are about 120° apart. Trigonal planar carbons are sometimes described as sp2 carbons.
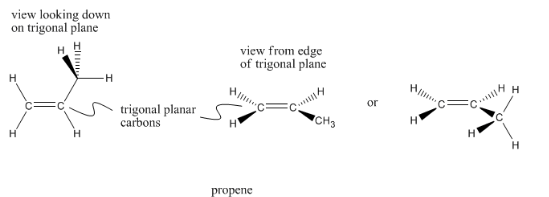
Propene has two trigonal planar carbons every bit well equally one tetrahedral carbon. Detect that a trigonal planar carbon is flat if viewed from one management, but not if viewed from the other. Sometimes you lot will encounter trigonal carbons drawn differently, if the drawer wants you to wait at the molecule from one direction or the other.
Encounter if you can detect both kinds of carbon on the model below.
Go to Animation IM9.ii. A three-dimensional model of propene.
Carbons that have only two different neighbors are linear; each neighbor is 180° apart from the other. Linear carbons are sometimes called sp carbons.
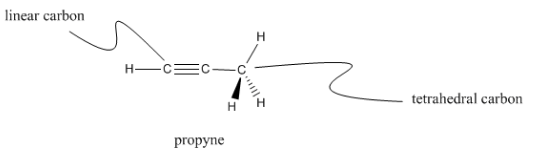
Propyne has ii linear carbons as well equally one tetrahedral carbon. See if yous tin notice both kinds of carbon on the model below.
Go to Animation IM9.3. A three-dimensional model of propyne.
Saying a carbon is sp or sp2 is only giving the shape of one atom within a molecule. The molecule itself may accept a particular shape which is determined by the geometries of it constituent atoms, but which tin can't be described simply in words as "tetrahedral" or "linear".
Pentane is roughly a zig-zag shape. In a space-filling model, information technology resembles a fat caterpillar inching forth. Propene is nearly boomerang-shaped. Propyne is shaped like a dart; it has a pointed end and a feathered end.
Other molecules have more than complicated shapes. The shape of ginkgolide B (a natural product isolated from the medicinally interesting ginkgo biloba tree) is difficult to describe. Even so, it is clear that individual carbons in ginkgolide B are mostly tetrahedral, with just a couple of trigonal planar ones.
Get to Blitheness IM9.four. A iii-dimensional model of ginkgolide B.
Understanding the overall shape of a molecule is particularly of import in diverse aspects of biochemistry. For example, the effect of whatsoever drug in the body is strongly influenced past the shape of the drug and how that shape tin interact with specific receptors.
Exercise \(\PageIndex{i}\)
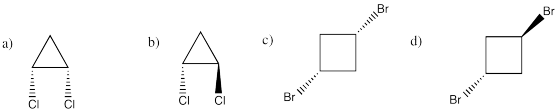
Cis and trans cyclic isomers take 2 substituents attached to a band. The cis isomer has both substituents on the aforementioned face of the ring. The trans isomer has substituents on opposite faces of the band. Use wedge-dash projections to draw the shapes of the following molecules:
- cis-1,two-dichlorocyclopropane, CthreeH4Cl2 (the numbers mean there is a chlorine on the start carbon and a chlorin on the second carbon)
- trans-1,2-dichlorocyclopropane, CiiiH4Cltwo c) cis-1,3-dibromocyclobutane, C4H6Br2 d) trans-1,3-dibromocyclobutane, C4Hhalf dozenBrii
- cis-1,3-dibromocyclobutane, C4H6Br2
- trans-1,3-dibromocyclobutane, C4Hhalf-dozenBr2
- Answer
-
Exercise \(\PageIndex{2}\)
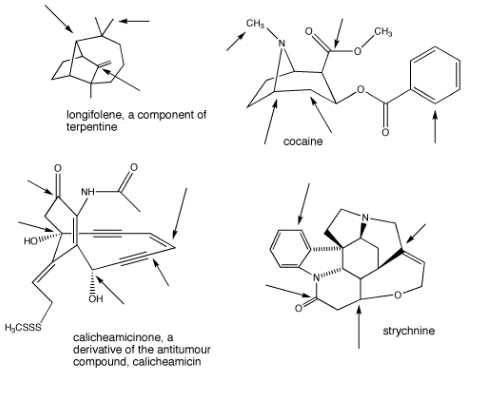
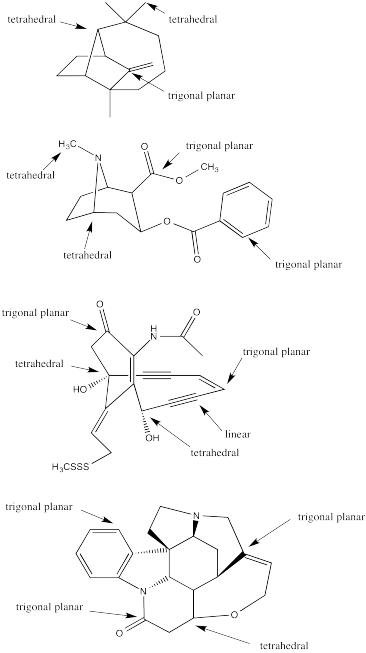
Identify the geometry at the indicated carbons in the post-obit molecules:
- Answer
-
How To Draw A Tetrahedral Molecule,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_Structure_and_Reactivity_in_Organic_Biological_and_Inorganic_Chemistry_(Schaller)/I%3A__Chemical_Structure_and_Properties/04%3A_Introduction_to_Molecules/4.09%3A_Three_Dimensional_Drawings
Posted by: williamsherat1979.blogspot.com


0 Response to "How To Draw A Tetrahedral Molecule"
Post a Comment